Oxidative Stress & The Foundations for Longevity: A Comprehensive Review
Aging involves the gradual degeneration of cells over time, leading to decreased physiological function, higher susceptibility to disease, and ultimately death.
As you age, your body goes through processes like cellular senescence, telomere shortening, and changes in gene expression.
These mechanisms are a normal part of human aging when they happen gradually. However, in modern times, we’re experiencing significantly accelerated rates of aging.
This means these natural processes are happening at younger ages than they should, leading to earlier cases of age-related disease.
Table of Contents
Oxidative Stress: A Major Cause of Aging
Today, although we see an increase in human lifespan over the last several decades, health span or quality of life is on the decline due to accelerated aging and the increased prevalence of age-related disease.1
Many things influence accelerated aging and age-related disease, but one phenomenon at the root of these detrimental mechanisms is oxidative stress.
In the fight for longevity, oxidative stress plays a major role, but before combatting it, we first must understand the scope of this complex phenomenon.
In this article, we’ll discuss the mechanisms involved in oxidative stress, its measurement, and its impact on aging and health using key findings from Vibrant research.
What is Oxidative Stress?
Oxidative stress is an imbalance between the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) and the body’s antioxidant defenses.
ROS and RNS are unstable compounds that can damage critical cellular components like lipids, DNA, RNA, and proteins.
The job of antioxidants is to manage these reactive species.
It’s normal for the human body to experience some oxidative stress—the issue is when there are excessive numbers of these compounds and not enough antioxidants to neutralize them.
This imbalance leaves the body in a state of overwhelming oxidative stress, which can harm virtually every system.

What Causes Oxidative Stress?
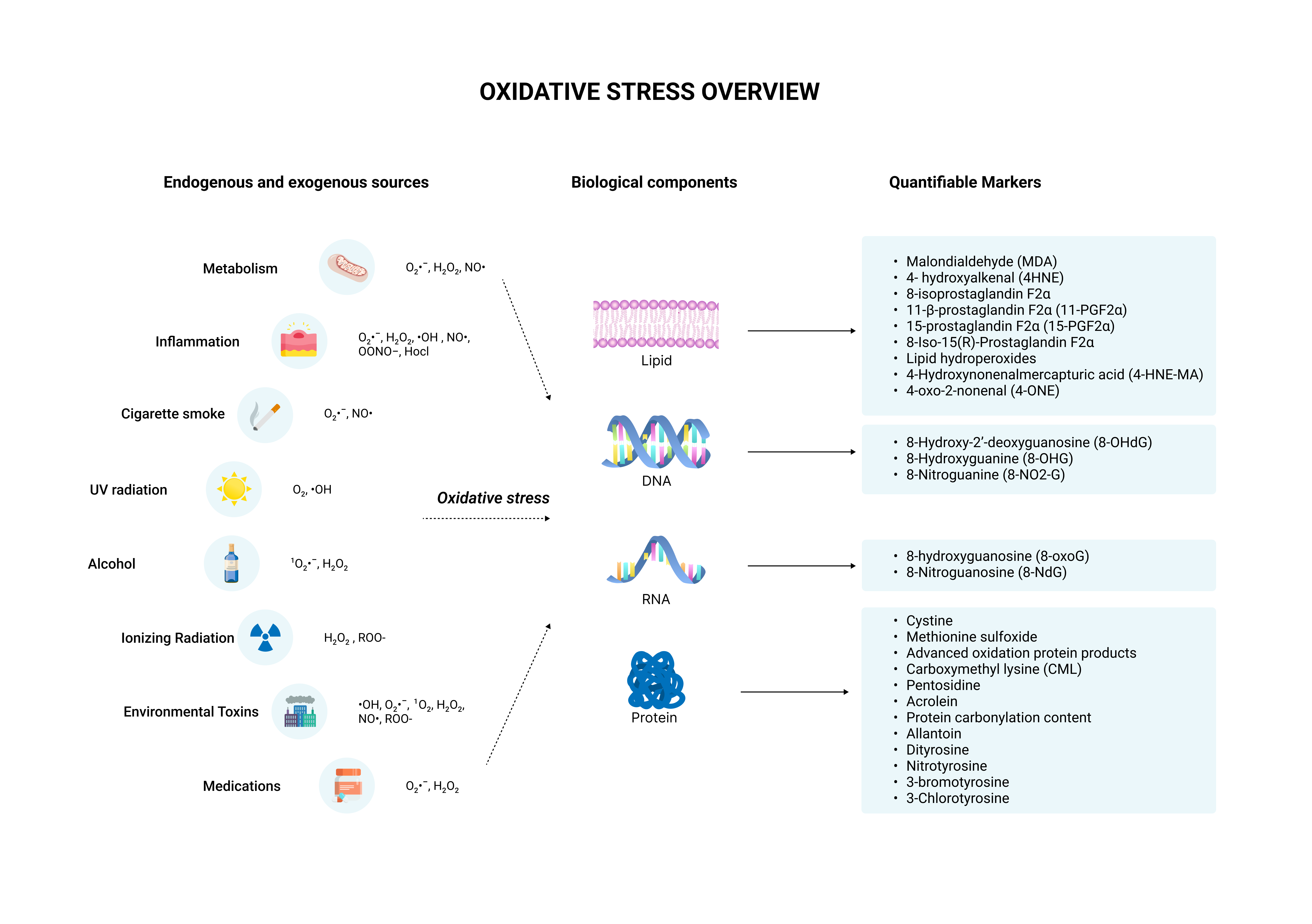
Both endogenous and exogenous factors can disrupt the balance of reactive species and antioxidants.
Endogenous sources include:
- Mitochondrial respiration
- Inflammation
- Enzymatic reactions
- Inadequate or ineffective natural antioxidant levels
Exogenous sources include:
- Phthalates
- Pesticides
- Pollution
- Heavy metals
- Medication
- Poor diet
- Cigarette smoke
Reactive Species: Radical vs. Non-Radical
ROS and RNS are essential by-products of cellular metabolism but can pose significant threats when their balance is disrupted.
ROS and RNS are collectively known as free radicals. Free radicals are molecules containing one or more unpaired electrons, making them unstable.
The unpaired electrons are readily available to react with organic substrates like lipids, proteins, and DNA, inflicting damage on these substances.
The body's primary source of free radicals is energy production processes in the mitochondria.2
Free radicals include the superoxide anion (O2•ˉ), hydroxyl radical (•OH), and nitric oxide (•NO).
However, not all oxygen and nitrogen species are free radicals. Some are known as non-radical species and can also damage foundational structures.
Non-radical species, such as hydrogen peroxide (H2O2) and peroxynitrite (OONO−), although not radicals themselves and not as reactive, can lead to the formation of free radicals or act directly to damage cellular structures.
Sources of ROS and RNS
Mitochondria stand out as the primary internal source of ROS through ATP production.
They contribute to about 90% of cellular ROS generated in the body.2
Reactions carried out by complexes I and III of the electron transport chain, along with other enzymatic reactions in cellular organelles like peroxisomes and the endoplasmic reticulum, contribute significantly to ROS generation.
Enzymatic reactions also play a central role in RNS production.
Further, immune reactions, among other mechanisms, can lead to the development of reactive species in the body.
Externally, various factors can influence ROS and RNS levels including ultraviolet radiation, pollution, cigarette smoke, alcohol consumption, and certain medications.
These sources introduce additional reactive species into the body, exacerbating oxidative stress when not adequately neutralized by antioxidants.
Free radicals can initiate lipid peroxidation, damaging cell membranes, while non-radicals can penetrate biological membranes and contribute to cellular damage indirectly.
The interplay between ROS and RNS, and the resulting production of highly reactive species, is the key driver of oxidative stress and its harmful impact.
Measuring Oxidative Stress

Direct Testing Methods
One method of determining oxidative stress levels is directly measuring ROS and RNS. However, this strategy poses significant challenges.
One direct method involves using specific fluorescent probes, such as DCFDA, for ROS like hydrogen peroxide (H2O2) and dihydroethidium (DHE) for superoxide (O2•ˉ). Although these probes offer a way to visualize and quantify reactive species, their application is marred by specificity and sensitivity issues.
For instance, DCFDA can react with various ROS, not just H2O2, making it non-specific. Further, local oxygen levels and pH can affect its fluorescence yield, complicating the interpretation of results.
Similarly, while DHE targets superoxide, its differentiation from other oxidative products is complicated due to overlapping fluorescence spectra, raising concerns about quantification accuracy.
Direct methods struggle primarily because ROS and RNS are highly reactive and short-lived; some species, like the hydroxyl radical (•OH), exist for less than a nanosecond before reacting with other cellular components.
This transient nature, combined with the relatively low levels of these species compared to other cellular components, makes their quantification difficult.
Complex Testing Techniques
You can quantify ROS using more complex techniques such as electron spin resonance, spin trapping, or pulse radiolysis. However, these methods can be labor-intensive, time-consuming, and may require sophisticated instrumentation, which limits their general use.
Given these challenges, research has shifted towards indirect methods, focusing on oxidative damage markers (e.g., lipid peroxidation products, protein carbonyls) and antioxidant levels.
These indirect markers provide a more reliable assessment of oxidative stress by measuring the aftermath of ROS/RNS activity or the body's defensive capacity against oxidative damage, circumventing the challenges associated with directly measuring reactive species themselves.
Precision Testing: The Oxidative Stress Profile

The best way to measure indirect markers of oxidative damage is through precision testing like Vibrant’s Oxidative Stress Profile.
This panel measures 16 markers of cumulative oxidative damage eliminated from the body in the urine, as well as 32 genetic variants that code for enzymes and antioxidants that can significantly impact the oxidative stress response.
This profile measures more analytes than any known oxidative stress test, providing robust insight into oxidative damage to DNA, RNA, lipids, and proteins.
It’s also the only test that pairs cumulative markers of oxidative damage with genetic predispositions toward oxidative stress, allowing you to create personalized treatment plans that support the body's natural antioxidants (through dietary sources) for optimal antioxidant creation and recycling.
The Oxidative Stress Profile also includes a unique Oxidative Damage Score, indicating speed of aging based on oxidative damage to vital structures, which can help guide treatment plans.
Beneficial Functions of Oxidant Species
Oxidant species, including ROS and RNS, are crucial in maintaining physiological balance within the body.
Contrary to the damage they can inflict at high levels, at low to moderate concentrations, these species are essential for various beneficial processes.
One example is how ROS help regulate cell growth and apoptosis2, the programmed cell death necessary to eliminate damaged or unnecessary cells. They accomplish this through interactions with transcription factors and signaling pathways, vital for cellular proliferation and survival.
Additionally, ROS play a significant role in the immune system. During a bacterial infection, immune cells like neutrophils and macrophages utilize ROS in a process known as the 'respiratory burst' to produce substances like hydrogen peroxide and hypochlorous acid, which are potent in combating pathogens.

ROS can even influence the expression of antioxidants in the body. Certain ROS are involved in pathways leading to the upregulation of genes that code for antioxidant enzymes and molecules, bolstering the body's defense against oxidative stress.
On the other hand, RNS, particularly nitric oxide (•NO), play indispensable roles in cell signaling, enzyme regulation, and the relaxation of smooth muscles in blood vessels— essential for regulating blood pressure.
•NO also contributes to the body's non-specific immune defense by helping destroy pathogens and tumors. Finally, the body’s continuous production of ROS and RNS through metabolic activities underlines their importance as vital contributors to human health when maintained at low or moderate levels.
This dual nature emphasizes the necessity of a balanced oxidative state for optimal physiological function and health.
Detrimental Effects of Oxidant Species
The detrimental effects of oxidant species, specifically when their levels surpass the body's neutralizing capabilities, manifest in several forms of oxidative damage to integral biological structures.
Oxidative stress leaves lipids, nucleic acids, and proteins open to attack, disrupting cellular integrity and function.
One example is lipid peroxidation, where reactive species attack lipids containing carbon-carbon double bonds, specifically polyunsaturated fatty acids (PUFAs).
This creates lipid radicals that react with other lipid molecules, forming a harmful chain reaction.
These radicals compromise membrane structure and function—the process decreases membrane fluidity and inactivates membrane-bound enzymes and receptors.2
Additionally, oxidative stress can significantly damage DNA and RNA, inducing mutations and base modifications that disrupt genetic information and integrity.

Proteins, too, are not spared—oxidative damage can induce protein structural and functional denaturation, causing loss of enzyme activity and disrupting cellular signaling and transport.
Among the oxidative damage products formed, advanced oxidation protein products (AOPP) emerge as a critical biomarker for oxidative stress indicating protein damage.2
Proteins can be modified through reactions with reactive oxygen species, leading to the formation of Amadori products or advanced glycation end products, which can further compromise protein function.
Amadori products act as crucial intermediates in the formation of advanced glycation end products (AGEs) through the Maillard reaction, where reducing sugars react with protein amino groups.
These products undergo further complex reactions to become AGEs, which are linked to various age-related diseases by altering protein structure and function. The progression from Amadori products to AGEs highlights their significant role in the detrimental effects of oxidative stress on protein function.
Antioxidants

Antioxidants are the body’s defense system against oxidative stress and are diverse in structure and function, enabling them to operate effectively in both hydrophilic and hydrophobic environments.
They are broadly categorized into enzymatic and non-enzymatic types, each playing a unique role in neutralizing free radicals and preventing cellular damage.
Enzymatic Antioxidants
Enzymatic antioxidants directly target ROS and RNS, acting as the first line of defense. Superoxide Dismutase (SOD) transforms the superoxide radical into hydrogen peroxide (H2O2) and oxygen, a crucial step in mitigating oxidative damage.2
Catalase (CAT) and Glutathione Peroxidase (GPx) further detoxify H2O2 by converting it into water and oxygen. GPx also reduces lipid hydroperoxides using glutathione as a substrate.
Secondary enzymes like the thioredoxin system, thioredoxin peroxidases, and glutaredoxins support the antioxidant defense by reducing oxidized forms of primary antioxidants and repairing proteins damaged by reactive species.
Non-Enzymatic Antioxidants
Non-enzymatic antioxidants, both endogenous and exogenous, complement the action of enzymatic antioxidants by directly scavenging reactive species.
Endogenous non-enzymatic antioxidants include:
- Glutathione: The most prevalent cellular thiol-based antioxidant
- Uric acid: Contributes significantly to plasma antioxidant capacity
- Albumin: Scavenges hydroxyl radicals; bilirubin, known for its lipid-protective effects
- Coenzyme Q10: Crucial for mitochondrial redox balance
- Melatonin: Crosses cellular barriers to protect DNA, lipids, and proteins
- Alpha-lipoic acid: Active in both aqueous and lipid phases and capable of regenerating other antioxidants
Exogenous antioxidants, obtained from the diet, include vitamins A, C, and E, selenium, zinc, and a wide range of polyphenols found in fruits, vegetables, and beverages. These contribute to the body's antioxidant defenses by scavenging ROS/RNS and chelating transition metals that could catalyze oxidative reactions.
These combined efforts of enzymatic and non-enzymatic antioxidants are essential for maintaining cellular health and preventing the detrimental effects of oxidative stress.
Oxidative Stress in Aging
While we can see the effects of oxidative stress in the short term, much of the damage accumulates over time and is directly linked to the aging process.
The Oxidative Stress Theory of Aging3 posits that aging results from the cumulative damage inflicted by reactive oxygen species (ROS) and reactive nitrogen species (RNS) on vital macromolecules such as lipids, DNA, RNA, and proteins.2
This damage can stem from increased oxidative stress, due to a decline in mitochondrial function, and the diminished efficacy of the body's antioxidant defenses over time.
As the efficiency of endogenous antioxidant systems wanes with age, possibly due to nutritional and hormonal changes, the balance shifts toward an accumulation of oxidative damage, marking a critical factor in the aging process.
This accumulation of oxidative damage is closely linked to cellular senescence, a state in which cells cease to divide but remain metabolically active, a pivotal aspect of aging.
Oxidative stress induces cellular senescence by causing DNA damage, accelerating telomere shortening, and activating growth arrest pathways.

Senescent cells, through the senescence-associated secretory phenotype (SASP), release inflammatory factors, contributing to the pathogenesis of various age-related diseases such as cardiovascular disease, chronic kidney disease, neurodegenerative disorders, macular degeneration, biliary diseases, and cancer.
This interplay between oxidative stress and cellular senescence represents a vicious cycle, further exacerbated by inflammation.
The "Oxidation Inflammatory Theory" of aging suggests that chronic oxidative stress and subsequent inflammatory responses disrupt bodily homeostasis, leading to increased morbidity and mortality in older adults.
Thus, this cycle of oxidative stress and inflammation plays a pivotal role in the aging process and the development of age-related diseases.
The Role of Damage Markers & Genetics in Assessing Oxidative Stress

Oxidative Damage Markers
The most accurate way to assess oxidative stress is by looking potential indicators of oxidative damage.
Vibrant‘s Oxidative Stress Profile assesses levels of various damage products, including Glutathione 4-hydroxynonenal (GS-HNE), a product of lipid peroxidation, serving as a sensitive indicator of lipid oxidative damage.
The panel also detects nitrative stress biomarkers like 8-Nitroguanosine to evaluate nitrogen-based oxidative modifications, which can significantly impact cellular functions and contribute to disease pathogenesis.
Along with damage markers, genetics can also be used to assess an individual’s susceptibility to oxidative stress.
Genetic Markers
Among the key genetic markers utilized in the Oxidative Stress Profile are antioxidant genetics such as GPX1, a gene encoding for Glutathione Peroxidase 1, an enzyme vital in reducing lipid hydroperoxides to their corresponding alcohols and free hydrogen peroxide to water. This plays a critical role in moderating oxidative stress within the cell.
Genetic variations play a significant role in an individual's ability to manage oxidative stress and their capacity for damage repair. Certain genetic predispositions can influence the effectiveness of the body's antioxidant defenses, potentially leading to increased vulnerability to oxidative damage.
By integrating genetic analysis into the Oxidative Stress Profile, we offer a nuanced understanding of how genetic factors may exacerbate or mitigate oxidative stress, allowing for personalized health interventions tailored to the genetic makeup of each individual.
Oxidative Stress, Health, & Longevity
The intricate dance between oxidative stress and the body's defensive mechanisms underscores a pivotal aspect of our health, significantly influencing the aging process and the development of various diseases.
Understanding and managing oxidative stress emerges as a crucial strategy for enhancing longevity and improving quality of life.
By utilizing tools like Vibrant’s Oxidative Stress Profile, you can help your patients gain an unparalleled understanding of their oxidative stress status and create personalized solutions to promote long and healthy life spans.
References
1. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018 Apr 26;13:757-772. doi: 10.2147/CIA.S158513. PMID: 29731617; PMCID: PMC5927356.
2. Krishnamurthy, H., Pereira, M., R, I., Jayaraman, V., Krishna, K., Wang, T., Bei, K., & Rajasekaran, J. J. (2024). Oxidative stress: Mechanisms, quantification and its role in human aging. ScienceOpen Preprints. https://doi.org/10.14293/pr2199.000699.v1
3. Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018;13:757-772. doi:10.2147/CIA.S158513
Regulatory Statement:
The general wellness test intended uses relate to sustaining or offering general improvement to functions associated with a general state of health while making reference to diseases or conditions. This test has been laboratory developed and its performance characteristics determined by Vibrant America LLC and Vibrant Genomics, a CLIA-certified and CAP-accredited laboratory performing the test. The lab tests referenced have not been cleared or approved by the U.S. Food and Drug Administration (FDA). Although FDA does not currently clear or approve laboratory-developed tests in the U.S., certification of the laboratory is required under CLIA to ensure the quality and validity of the tests.
 By
By




