What Are Antibodies and Why Are They Important in COVID-19 Testing?
In the midst of the COVID-19 pandemic, as accurate testing for the virus is in short supply across the globe, there is a significant opportunity to provide information to patients and healthcare providers about exposure and infection timelines through antibody testing.
To add to the problem, nasopharyngeal (NP) swabs – considered the gold-standard for diagnosing the infection – which are tested for RT-PCR (a type of RNA test for the viral genetic material) are notoriously inaccurate when collected and stored/transported improperly, and can average an accuracy rate of only 63.45% when collected within the first 14 days after infection and that can drop to only about 52% accurate after day 14. [1]
That means that NP swab alone misses an average of 36.5-48% of patients with COVID-19 infections, depending on what time-point in the infection they are tested…that’s almost half!
Perhaps it is time to include additional testing methods in the standard diagnostic procedures for COVID-19 infections. And antibody tests may just be the answer to this problem. But, you may be unfamiliar with what antibodies are, and why they are potentially the most valuable tool in a healthcare provider’s toolbox during this time.
Also, as we will see, not all antibody tests are created equal.
Types of Antibodies
There are five main types of antibodies, also referred to as immunoglobulins, in the human body that are relevant to disease, infections, or inflammatory response. This article will deal only with the three that are considered definitely relevant to the COVID-19 viral infection.
The two most well-studied antibody types specific to COVID-19 are IgG and IgM. At this time, Vibrant America is the only laboratory actively investigating the role that IgA antibodies may play in diagnostic utility for COVID-19 immune responses, more about that later.
IgG
IgG antibodies are the most abundant in your blood, making up approximately 75% of the total amount of immunoglobulins/antibodies your body has floating around at any given time.
There are four sub-types of IgG antibodies, each with their own slightly different mechanism of action (aptly name IgG1, IgG2, IgG3, and IgG4).
IgG antibodies look like this:
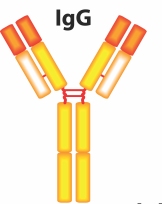
These antibodies have mechanisms that allow them to perform the following actions:
- initiating phagocytosis via macrophages (phagocytosis is ‘cell’ ‘eating’ – referring to the ability of macrophages to essentially engulf a target cell such as bacterial or viral cells and destroy them)
- agglutination (IgG antibodies ‘clump’ to an antigen such as a virus or other foreign body/protein and create a glob-like mass that is then usually phagocytosed by macrophages
- antibody-dependent cell-mediated cytotoxicity – the IgG antibody activates natural killer cells (NK) to target and neutralize a cell, such as a tumor cell or virus using cytotoxic compounds
- neutralize toxins – toxins come from other organisms such as venom or endotoxins from bacteria. IgG antibodies grab these at their active site so that the toxin cannot bind to any other cells or proteins
- activating complement – an additional inflammatory response cascade to foreign antigens
IgG antibodies are relatively small in size and are the only antibody that crosses the placental barrier, conferring passive immunity to newborns in the days and weeks immediately following birth.
Specific to COVID-19
This study from China, published Apr 10, 2020 looked at 67 patients diagnosed with COVID-19 through either nasopharyngeal (NP) swab RT-PCR or chest x-ray and found that when antibodies were measured on a colloidal gold-based immunochromatographic (ICG) strip assay (one of the tests referred to as ‘rapid tests’) showed that sensitivity of IgG antibodies was:
- 3.6% in patients within 1-7 days of infection
- 57% in patients within 8-14 days of infection
- 96.8% in patients at 15 days of infection
- a cumulative average of 54.7% sensitivity of antibody detection of patients with confirmed infection
In comparison, Vibrant’s ImmuneCheck COVID-19 Antibody assay was found to have the following:
- 340 total samples were tested for 4 antigens (S1 Spike protein, (RBD)Receptor Binding Domain, S2 Spike protein, Nucleoprotein) for the presence of IgG antibodies
- a cumulative average of 94.29% sensitivity of IgG antibody detection of patients with confirmed infection
The main antibody involved in the antiviral response is the IgG antibody. [2]
This study from China, conducted in Jan-Feb 2020 followed 23 patients with diagnosed COVID-19. With respect to viral shedding, they found:
- Anti-SARS-CoV-2-NP or anti-SARS-CoV-2-RBD IgG levels correlated with virus neutralization – reduced viral load = reduced IgG antibody levels = increased viral shedding
From that we can extrapolate that as IgG levels fall or begin to return toward baseline, viral load may be declining or have declined, and contagious status may be reduced.
This also highlights the importance of testing patients at multiple time-points of infection, if an initial test finds positive antibody levels. Without a retest of the patient at least 10-14 days after the initial positive antibody result, it may be difficult to determine at what point the patient has overcome the infection.
IgM
IgM antibodies have antiviral action, among other mechanistic purposes. They are considered an acute antibody, meaning they are one of the first antibody types to elevate in response to an infection, and generally only last approximately 5-10 days after initial presentation of their response (their half-life is considered to be 5 days).
IgM antibodies look like this:
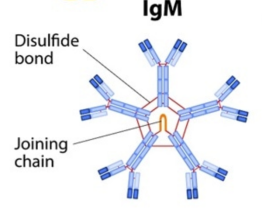
They are generally not detected after an infection is no longer active, usually once it is not ‘contagious’ by most medical definitions, however, we do not yet have very solid data on this specific to COVID-19, but that is the prevailing theory among researchers at this time.
However, because IgG antibodies are the primary antibody associated with viral neutralization and infection resolution [3], it is not yet known if IgM antibody levels can be used to definitely determine infectious course.
Some important functions performed by IgM antibodies are:
- activating complement
- agglutination of antigens – just like IgG antibodies can form a ‘clump’-like structure with an antigen in order to render it inert and then allow macrophages to destroy it, IgM antibodies can do this as well
Specific to COVID-19
This same study from China, above, which looked at 67 patients diagnosed with COVID-19 through either nasopharyngeal (NP) swab RT-PCR or chest x-ray and found that when antibodies were measured on a Colloidal gold-based immunochromatographic (ICG) strip assay showed that IgM antibodies were found in:
- 11% of patients within days 1-7 of infection
- 78% of patients within days 8-14 of infection
- 74% of patients past day 15 of infection
- a cumulative average of 55% sensitivity of antibody detection of patients with confirmed infection
In contrast, when we look at Vibrant’s ImmuneCheck COVID-19 antibody test, we see some really remarkably different results, due to the heightened sensitivity and specificity of the chemilluminescent silicon microarray platform:
- 340 total samples were tested for 4 antigens (S1 Spike protein, (RBD)Receptor Binding Domain, S2 Spike protein, Nucleoprotein) for the presence of IgM antibodies
- a cumulative average of 91.43% sensitivity of IgM antibody detection of patients with confirmed infection
IgA
IgA antibodies are also involved in the antiviral response when it is present in mucosal surfaces. Mucosal surfaces are found throughout the body in:
- the gut
- the lungs
- the nasopharyngeal tract
- the genital tract
IgA antibodies can also agglutinate antigens, just like IgM and IgG antibodies.
IgA antibodies look like this (this is a dimeric form of IgA, which is its most common form in the blood, but it can also exist as a monomer):
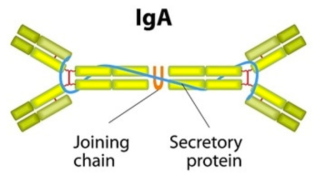
Specific to COVID-19
Because a primary target of the COVID-19 virus is the alveoli of the lungs, which are a major mucosal surface, it would stand to reason that IgA antibodies may have some relevance or diagnostic utility.
Vibrant America has included this antibody on its ImmuneCheck COVID-19 panel as an investigative assay to determine if this antibody should be included in standard antibody assessments of immune response to this virus.
So far, data about IgA antibodies to COVID-19 is limited or non-existent, but Vibrant’s validation study found that this antibody is:
- overall 65.7% sensitive to detecting exposure to the COVID-19 virus across four different antigens tested: SARS-CoV-2 Nucleoprotein (NP), SARS-CoV-2 Spike Glycoprotein (S1), SARS-CoV-2 Spike Glycoprotein (S2), SARS-CoV-2 Receptor Binding Domain
Combining IgG, IgM, and IgA antibodies across all four known antigens for COVID-19 yields a total cumulative test accuracy as below:
-
97.1% sensitivity (how frequently Vibrant’s test detects the COVID-19 virus when it is known to be present)
-
98.3% specificity (how frequently Vibrant’s tests correctly identifies the COVID-19 virus as opposed to detecting another similar virus that is not COVID-19 and reporting a false positive)
If you are a patient or healthcare provider interested in the Vibrant America ImmuneCheck COVID-19 test, click here.
References
Gary R. Klimpel. Medical Microbiology. 4th edition.Baron S, editor.Galveston (TX): University of Texas Medical Branch at Galveston; 1996
Kelvin Kai-Wang To, MD, Owen Tak-Yin Tsang, FRCP, Wai-Shing Leung, FRCP, Anthony Raymond Tam, MRCP, Tak-Chiu Wu, FRCP, David Christopher Lung, FRCPath, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. The Lancet, Infectious Diseases, Published:March 23, 2020, DOI:https://doi.org/10.1016/S1473-3099(20)30196-1
Pan Y, Li X, Yang G, Fan J, Tang Y, Zhao J, Long X, Guo S, Zhao Z, Liu Y, Hu H, Xue H, Li Y. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect. 2020 Apr 10. pii: S0163-4453(20)30175-4. doi: 10.1016/j.jinf.2020.03.051. [Epub ahead of print]
Yang Yang, Minghui Yang, Chenguang Shen, Fuxiang Wang, Jing Yuan, Jinxiu Li, Mingxia Zhang, Zhaoqin Wang, Li Xing, Jinli Wei, Ling Peng, Gary Wong, Haixia Zheng, Mingfeng Liao, Kai Feng, Jianming Li, Qianting Yang, Juanjuan Zhao, Zheng Zhang, Lei Liu, Yingxia Liu. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv, BMJ Yale. https://doi.org/10.1101/2020.02.11.20021493