The Surprising Role of Genetics in Detoxification
In the modern world, toxic exposure is ubiquitous and unavoidable. Our daily, excessive exposure to toxins can often lead to an excessive toxic burden—a state in which the body is overwhelmed with toxins.
When overwhelmed by toxins, the body attempts to excrete harmful substances through detoxification. But not everyone detoxes successfully. In fact, some of us are genetically prone to defective detoxing.
Toxic burden can further inhibit the body’s detox system because toxins trigger elevated oxidative stress and increase demand on the body’s detoxification pathways, particularly in the liver.
When the liver can’t adequately eliminate toxins or infectious byproducts, these toxins can clog normal detox pathways and lead to a range of chronic inflammation symptoms.
In this article, we’ll discuss the role of detox genetics, the effects of impaired detoxification, and how you can combine nutrition and lifestyle strategies with precision lab testing to create personalized detox solutions for your patients.
What Are Detox Genetics?
Detox genetics are specific gene variations that can affect how sensitive our bodies are to toxins that come from inside (endogenous) or outside (exogenous) sources, and how well we can remove those toxins.
There are certain single nucleotide polymorphisms (SNPs) that impact the function of detox enzymes and how dietary factors interact with those enzymes.
Some of these SNPs lead to an inability to detox substances efficiently, while others cause individuals to detox too quickly.
The specific genes that exert influence on the detoxification process can be narrowed down to genes that code for enzymes involved in Phase I and Phase II detoxification.
Phase I Detox Genes
Cytochrome P450 enzymes
Phase I detoxification involves the activation of specific enzymes, one major group involved is known as the superfamily of cytochrome P450 enzymes or CYP450. These enzymes use oxygen to metabolize toxins and synthesize molecules like cholesterol and steroids.
When carrying out these reactions, the Phase I enzymes create reactive substances that, if not processed in Phase II, can cause oxidative damage to the body.
CYP450 enzymes are crucial in drug metabolism. An individual’s ability to metabolize 90% of currently used drugs is largely determined by the genetic expression of these enzymes.
One gene in this family that codes for an influential Phase I enzyme is the Cytochrome P-450 1A2 (CYP1A2) gene. When detoxing, the CYP1A2 enzyme activates certain chemicals found in cigarette smoke, car exhaust, and charbroiled foods, turning them into carcinogens.
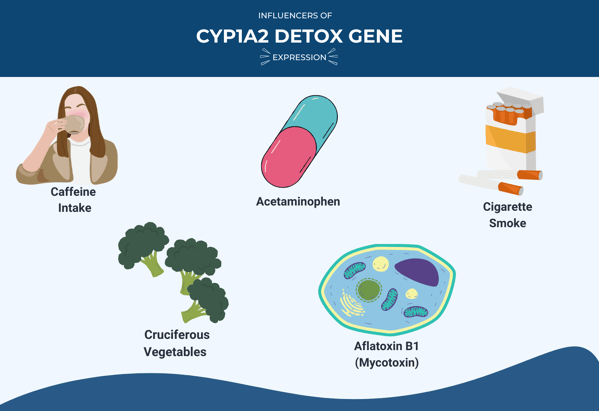
The expression of the CYP1A2 gene can be influenced by several factors, including:
- Caffeine intake
- Cruciferous vegetables
- Acetaminophen
- Aflatoxin B1 (a mycotoxin found in some foods)
- Cigarette smoke
Additionally, there is a genetic variation called rs762551 that can affect how the CYP1A2 enzyme is produced in response to caffeine or cigarette smoking.
People with the rs762551(C) allele metabolize toxins more slowly, while those with the rs762551(A) allele metabolize them faster. Those with the slower-metabolizing variant of the enzyme have significantly lower enzyme activity, and their enzymes are less responsive to the toxins that trigger their activity.
Consequently, having lower activity of the CYP1A enzyme can reduce the body's ability to detoxify harmful substances and increase the risk of certain cancers.
On the other hand, if the enzyme is too active without proper clearance, it can lead to the accumulation of highly reactive substances, causing more oxidative damage and increasing the risk of cancer.
Thus, genetic variations in Phase I detoxification genes can influence the speed at which toxins are converted and the efficiency of subsequent detoxification steps.
Phase II Detox Genes
The SULT Gene Group
Sulphotransferase 1A1 (SULT1A1) plays a major role in the bioactivation and detoxification of procarcinogens (substances that can potentially cause cancer), toxins, and foreign substances through sulfation— the process of adding a sulfate group to a molecule or compound.
This SULT gene group is responsible for the sulfation of endogenous molecules like steroid hormones and the elimination of xenobiotic (foreign) molecules such as drugs, environmental chemicals, and natural products.
Genetic polymorphisms and copy number variations in the SULT1A1 gene can alter the activity and thermostability of the enzyme it codes for.
These genetic variations can make individuals react negatively to certain medications and more susceptible to hormone-dependent cancers.
 One such medication is Tamoxifen, an estrogen receptor modulator commonly used to prevent and treat breast cancer.
One such medication is Tamoxifen, an estrogen receptor modulator commonly used to prevent and treat breast cancer.
Increased risk of cancer recurrence and death in tamoxifen-treated patients has been shown in those with variations in SULT1A1 alleles.
Two notable genetic variants of the SULT1A1 gene include SULT1A1*2 and SULT1A1*3.
Additionally, genetic variants of the SULT genes are linked to Parkinson’s Disease— patients with the disease demonstrate a deficiency in the detox pathways of sulfur metabolism.
Thus, variations in the SULT genes interfere with sulfation and enzyme activity, and this can lead to impaired drug and hormone metabolism and detoxing ability, increasing the development risk of certain ailments such as breast cancer.
Detox Genetics Health Implications
Some individuals may possess gene variants that enhance the activity of detox enzymes, making them rapid detoxifiers, while others have variants associated with reduced enzyme activity, resulting in slower detoxification.
Interestingly, both scenarios can have implications for an individual's health. Rapid detoxifiers may have a heightened ability to eliminate toxins efficiently, but they might also be more susceptible to adverse reactions caused by oxidative stress and an increased risk of diseases like cancer when exposed to certain medications or environmental toxins.
On the other hand, slow detoxifiers may have a decreased ability to eliminate toxins effectively, potentially increasing their susceptibility to toxin-related health conditions.
Body Systems Affected by Impaired Detoxing

One’s ability to detox and process harmful substances is an integral part of maintaining optimal health and preventing disease. Toxins come from various sources, including the environment, food and water, and medications—our bodies are in a constant state of exposure and detoxification.
If the body can’t efficiently remove these substances, it can impact several organ systems. including:
- Liver and kidneys
- Central nervous system
- Cardiovascular system
- Skin
- Endocrine and reproductive systems
The liver is the major detoxification organ in the body and where most detoxing takes place. If detoxification is impaired, highly-reactive free radicals (that are produced as part of the normal detoxing mechanism) will not be processed and can damage cell structure and function in the liver.
Too many free radicals can cause oxidative stress to the body, and this can damage virtually all organ systems.
One example is the central nervous system. Oxidative stress can impact the biochemical functioning of the brain, as the brain is highly susceptible to it. This can impact normal central nervous system functions and is linked to neurodegenerative disorders like Alzheimer’s and Parkinson’s.
Defective Detoxing & Toxic Burden
![[BLOG HEADER] PFAS eBook](https://blog.vibrant-wellness.com/hs-fs/hubfs/%5BBLOG%20HEADER%5D%20PFAS%20eBook.png?width=749&height=499&name=%5BBLOG%20HEADER%5D%20PFAS%20eBook.png)
Impaired detoxification also triggers toxic burden, which can manifest in multiple organs, tissues, and cellular-level systems.
A high toxic burden can cause hyperintestinal permeability, also known as “leaky gut,” leaving the gut compromised and susceptible to additional issues like bacterial imbalance, food sensitivities, chronic inflammation, and increased risk of disease.
Additionally, many toxins, such as per- and polyfluoroalkyl substances (PFAS) bioaccumulate and persist in the body for decades, breaking down slowly or not at all.
Without effective detoxification, this chronic state of toxicity (high toxic burden) will leave individuals more susceptible to other toxic build-ups, infections, and diseases.
Finally, defective detoxing and toxic burden can have a causal relationship. When a patient is in a state of high toxic burden, the toll on the body can cause compromised immunity, increased oxidative stress, and impaired detoxification. This results in a vicious cycle of chronic toxic overload and defective detoxing.
Conditions Associated with Impaired Detoxification
An impaired detoxification system is linked to a wide range of conditions and chronic diseases, including cancer, asthma, obesity, cardiovascular disease, diabetes, and neurological conditions like Alzheimer’s.
Detox genetics can influence the risk of developing these diseases because genes code for specific enzymes that power the detoxification process, and when the activity level of these enzymes is not what it should be, the body may only partially detox harmful substances—or fail to remove toxins altogether—leaving behind dangerous free radicals.
Altered enzyme activity can cause a range of issues in the body:
- Low CYP1A enzyme activity can result in reduced toxin excretion, imbalanced estrogen metabolites, and a higher risk of certain cancers
- Low activity of the CYP1A2 enzyme is linked to an increased risk of developing testicular cancer
- Deletions in the GSTM1 genes can cause a lowered ability to detoxify environmental toxicants, carcinogens, and products of oxidative stress
Other detox genetic variations can lead to additional problems, including an increased risk of developing neuropsychiatric disorders and increased sensitivity to specific drugs like acetaminophen.
Disease States & Toxic Overload
![[BLOG HEADER] PFAS eBook (1)](https://blog.vibrant-wellness.com/hs-fs/hubfs/%5BBLOG%20HEADER%5D%20PFAS%20eBook%20(1).png?width=2160&height=1440&name=%5BBLOG%20HEADER%5D%20PFAS%20eBook%20(1).png)
To add to this, impaired detoxification leaves the body susceptible to conditions and diseases that are triggered by high toxic exposure. One example of this is autoimmune disease.
Many studies have shown that environmental toxins like pollutants, toxic metals, solvents, and endocrine disruptors have played a major role in the progression of autoimmune disease.
Exposure to chemicals can cause immune dysregulation and autoimmune reactivity in those who are genetically susceptible.
Chronic conditions like chronic fatigue syndrome, or myalgic encephalitis, are also linked to toxic exposure, specifically mycotoxins. In cases of chronic fatigue syndrome, mycotoxins may be behind the mitochondrial dysfunction associated with the condition.
Chronic fatigue syndrome has many possible causes, including infections (particularly by viruses) and oxidative stress. Keep in mind that inefficient detoxing can also lead to oxidative stress and increased susceptibility to infection. Thus, in a multi-faceted way, defective detoxing can trigger the development of chronic fatigue syndrome and similar conditions.
To prevent the development of these diseases and toxic overload, it's essential that the body’s detoxification system is functioning properly.
Precision Testing Options for Detox Genetics
The best way to fully understand a patient’s detoxing ability and the current toxic burden is to combine toxin testing with genetic testing.
The Total Tox Burden test detects levels of heavy metals, mycotoxins, and environmental toxins in the body, giving you insights into your patient’s total toxic burden.
The Toxin Genetics Panel detects the SNPs involved in Phase I and Phase II of detoxification, so you can assess your patients’ genetic predisposition to defective detoxing.
Toxin Genetics tests for markers such as CYP1B1 and CYP1A2 to assess detox enzyme function.
This genetic data will reveal how well your patients can detox harmful substances, and if they are at greater risk of developing high toxic burden and related diseases.
Combining these two panels provides the most holistic and intricate view of detoxification, and will help you create extensive, individualized treatment plans for patients.
Functional Medicine Treatment Options for Managing Detox Impairment & High Toxic Burden
Although exposure to toxins is constant, there are numerous strategies your patients can implement to promote detoxing and reduce toxic burden. First, it’s important to understand the different phases of detoxification.
Phases of Detoxification
There are three phases of detoxification in the body:
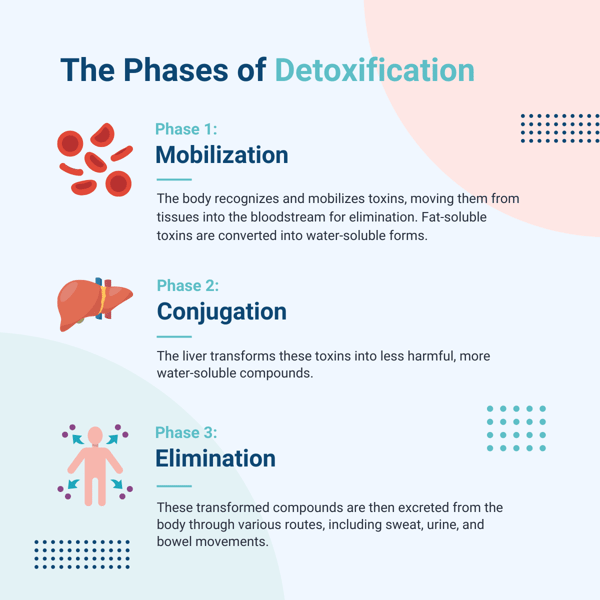
1. Functionalization involves altering the toxic compounds in your body by adding reactive sites with the help of enzymes.
The purpose of this reaction is to make the toxic compounds more hydrophilic, or water-soluble. The reactions turn these compounds into small molecules called intermediary metabolites and create reactive oxygen species in the body as a byproduct, which are addressed and detoxed in phase three.
2. Conjugation involves adding a water-soluble group (endogenous hydrophilic substance) to the reactive site on the new molecule formed in phase one. Many different hydrophilic compounds, using different enzymes, catalyze this step of detoxification.
Phase two aims to make the metabolite more water soluble, increasing the body’s ability to eliminate the substance.
3. Excretion involves the removal of toxins from the body through urine, stool, and the skin.
The 5-R Protocol
.png?width=1080&height=675&name=Antioxidant%20(7).png)
One recommended approach to aid in detoxification is the 5-R Protocol. This five-step method supports the body’s natural detoxification mechanisms, provides support for all three phases of detoxification, and improves gut health which directly affects detoxing ability.
Step 1: Remove the source of toxicity by identifying it, and reducing or eradicating exposure. This can include environmental toxins, such as mycotoxins, heavy metals, and chemicals like PFAS.Additionally, patients should remove gut irritants from their diets to promote gut function and detoxification. These include caffeine, alcohol, and inflammatory foods like dairy, gluten, and high-mercury seafood.
Patients should also wash produce with baking soda, including organic produce, to help remove toxins from the diet, and avoid packaged foods to minimize exposure to toxins commonly found in plastic packaging.
Step 2: Replace irritants with nutrients that support the three phases of detox in the body. Some examples include nuts and seeds like hazelnuts and flax seeds and herbs and spices like garlic and ginger.
Soak and sprout grains, legumes, nuts, and seeds before cooking or consuming them to help increase nutrient absorption.
Step 3: Repopulate the gut with helpful bacteria by including prebiotic and probiotic-rich foods such as fermented vegetables, kombucha, and kefir (non-dairy).
Step 4: Restore the gut to optimal health by focusing on anti-inflammatory foods and key nutrients such as antioxidants. Choose organic, non-GMO foods to reduce exposure to environmental pesticides, herbicides, and fungicides.
Step 5: Rebalance your gut by maintaining long-term health and preventing future toxin buildup. Make lifestyle adjustments in areas like sleep, exercise, and stress to support consistent detoxification.
Access the full Detoxification Food Plan to give your patients an in-depth guide to what foods they should be eating, and which to avoid, to promote detoxification.
As we’ve learned, limiting exposure to toxins may not be enough for those genetically prone to defective detoxing.
By combining nutrition and lifestyle strategies with advanced detox testing, you can help your patients assess detox ability, determine their levels of toxins and risk of developing a high toxic burden, and get to the root of chronic symptoms.
Regulatory Statement:
The general wellness test intended uses relate to sustaining or offering general improvement to functions associated with a general state of health while making reference to diseases or conditions. This test has been laboratory developed and its performance characteristics determined by Vibrant America LLC and Vibrant Genomics, a CLIA-certified and CAP-accredited laboratory performing the test. The lab tests referenced have not been cleared or approved by the U.S. Food and Drug Administration (FDA). Although FDA does not currently clear or approve laboratory-developed tests in the U.S., certification of the laboratory is required under CLIA to ensure the quality and validity of the tests.
 By
By





