Research Spotlight: Protein Microarray for Comprehensive Tickborne Testing
Did you know tickborne infections are the most common vector-borne diseases in the US?
While Lyme disease is the most common tickborne infection in the US and Europe, there are many other infections ticks can carry and spread to humans.
Tickborne infections can be difficult to diagnose due to:
- A lack of awareness about tick populations
- Inadequate testing
- Symptoms that mimic other conditions
These factors can lead to misdiagnosis and further health complications.
The diagnostic tests currently available for tickborne diseases are severely limited in their ability to provide accurate results and can’t detect multiple pathogens at once.
In this article, we’ll explore the necessity of multi-pathogen biomarkers, highlight breakthrough research underscoring this need, and introduce how Vibrant’s Tickborne Diseases panel leverages first-of-its-kind technology to redefine testing standards.
Why Testing for Multiple Pathogens Matters
Over the past few decades, tick populations and the diseases they carry have surged—more than doubling in the past 13 years.
According to the US Department of Health and Human Services, there are currently 20 different tickborne infections in the US, including Lyme, babesiosis, and ehrlichiosis.
Yet patients are rarely tested for all possible infections ticks could transmit through a bite.
This is because current diagnostic tests struggle to distinguish the many types of tickborne infections. According to several studies, non-Lyme tickborne infections are heavily underdiagnosed.
Recent research reveals that the standard two-tier testing recommended by the CDC can lead to false positive/negative results. The ELISA and Western blot tests can miss up to 60% of well-defined Lyme disease cases.
Regardless of diagnosis, ticks can and do transmit many infections in one bite, leading to prolonged exposure to Lyme and its co-infections.
Furthermore, standard treatments for tickborne diseases typically involve prolonged courses of antibiotics, which can disrupt the gut microbiome and compromise the immune system, leave patients at risk of developing:
- Multiple chronic inflammatory symptoms or conditions
- Additional infections like Epstein-Barr virus and opportunistic pathogens
This means that tickborne tests must test for a broad array of pathogens to be fully effective.

The Vibrant Tickborne Diseases Panel
The Vibrant Tickborne Disease Panel tackles this issue, detecting a broad range of antibodies and exposure to multiple pathogens simultaneously.
Vibrant’s Tickborne panel provides protein microarray antigen and PCR detection of Lyme disease and TBRF and co-infections of tickborne diseases like Anaplasma, Babesia, Borrelia, Bartonella, Ehrlichia, and Rickettsia species.
The panel’s dual approach—testing for antibodies (indirect) and DNA (direct) — provides the most comprehensive Lyme and co-infection detection.
Research Study: Customizable Protein Microarray Technology
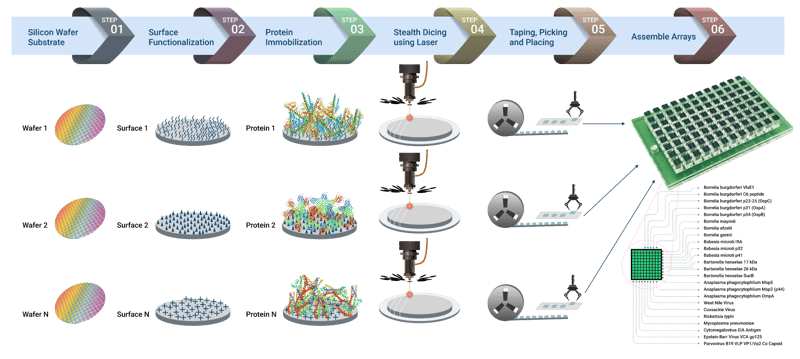
“A customizable multiplex protein microarray for antibody testing and its application for tickborne and other infectious diseases” is a 2023 study published in Research Square.
The Vibrant research team collaborated with over 25 renowned global researchers, including the CDC, NY Department of Health Parasitology Laboratory/Wadsworth Center (NYSDOH), universities, and private clinics to measure the Vibrant Tickborne panel’s effectiveness at detecting various tickborne and related pathogens.
The Method
The study took 2990 individuals with various tickborne diseases, totaling 87 different antigens, and collected their serum samples. The goal was to test Vibrant’s Tickborne panel for major performance metrics, including precision, sensitivity, reportable range, linearity, and matrix equivalency studies.
The Vibrant Tickborne disease panel tests for IgG and IgM antibodies for Lyme disease and other infectious agents.
Researchers analyzed the IgM and IgG immune responses of the individuals tested to determine the clinical sensitivity and specificity, among other metrics, of the panel.
The Technology
Vibrant’s Tickborne Diseases panel uses a first-of-its-kind customizable multiplex protein microarray and chemiluminescence detection to test antibody reactions to pathogens.
The main components of the immunochip platform include:
- Silicon-based microchips that are laser-diced from antigen-immobilized wafers
- A customizable 24-well compatible plate containing 24 pillars, each with 100 microchips
- A high-resolution imager capable of detecting chemiluminescent signals from labeled antigen–antibody reactions at each microchip
When compared with traditional testing methods recommended by the CDC, Vibrant’s technology boasts many advantages, including:
- Optimization: Allows testing for many different antigens at the same time, without sacrificing accuracy or sensitivity
- Automation: The automated process enhances efficiency
- Utilization of Resources: Reduces turnaround time and labor costs and removes the need for manual handling and subjective result interpretation
- Streamlined Process: Detects all antibodies in a single run
Each chip functions like an individual band in a Western blot test. However, the proteins are physically separated. This eliminates the cross-reactivity often seen in blot-based tests for proteins with similar mass.
To test antibody reactions to the tickborne antigens, a single serum sample is applied to each pillar in the assay, testing against all antigens at the same time.
The tickborne panel can test 192 individual samples in just 2 hours.
Vibrant labs also created an in-house IgM assay to increase detection of IgM antibodies. These antibodies normally only account for 5-10% of all circulating antibodies. Still, they are the primary antibodies produced by the immune system during infection and the ones we want to detect on the tickborne panel.
The IgM assay accomplishes this by removing IgG antibodies and other non-specific proteins from the serum before IgM immunoassay testing. This allows for enhanced detection of IgM antibodies.
Results & Clinical Implications
The 87 different antigens tested produced a specific immune response (IgM and IgG antibodies), which researchers compared to the immune response from the controls.
The study demonstrated that immunochip array technology outperformed traditional gold standard testing methods such as Western blot on clinical sensitivity and specificity, achieving up to 100% accuracy for multiple pathogens.
What This Means for Practitioners
With protein microarray technology, you can access the full scope of antigen reactions to make a nuanced diagnosis for your patients—rather than relying on a limited number of antigen reactions available.
By catching infections earlier, you can tailor treatments more effectively, reduce the risk of chronic symptoms, and prevent the long-term complications associated with tickborne diseases. Vibrant’s Tickborne panel enables you to make informed decisions, backed by precise and comprehensive data.
About the Author: Hari Krishnamurthy is the Director of Biomedical Engineering at Vibrant Labs. He strives to use his background in semiconductors and microchips to make a meaningful impact on healthcare.
Regulatory Statement:
The general wellness test intended uses relate to sustaining or offering general improvement to functions associated with a general state of health while making reference to diseases or conditions. This test has been laboratory developed and its performance characteristics determined by Vibrant America LLC and Vibrant Genomics, a CLIA-certified and CAP-accredited laboratory performing the test. The lab tests referenced have not been cleared or approved by the U.S. Food and Drug Administration (FDA). Although FDA does not currently clear or approve laboratory-developed tests in the U.S., certification of the laboratory is required under CLIA to ensure the quality and validity of the test
 By
By



